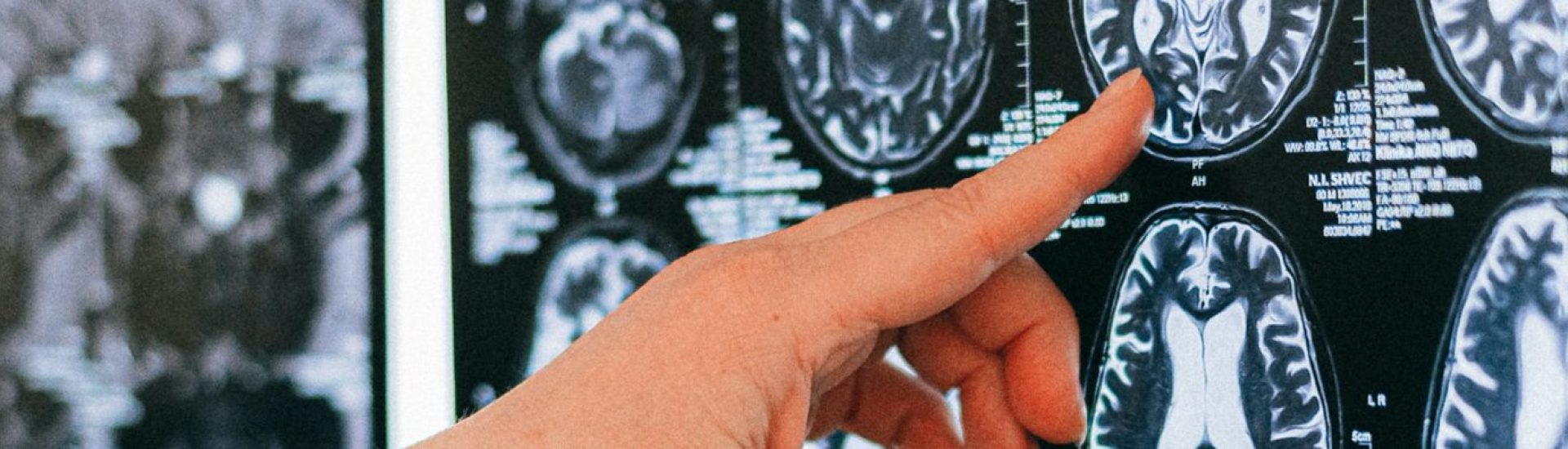
Following its initial announcement in May, the trial of the Alzheimer’s drug donanemab has yielded highly encouraging preliminary results, which have now been confirmed. This antibody medicine demonstrates its efficacy in the early stages of the disease by effectively clearing a specific protein that accumulates in the brains of individuals suffering from this form of dementia.
While it’s essential to clarify that donanemab is not a definitive cure, its impact has been significant enough to garner attention from various charities, heralding a new era in Alzheimer’s treatment. These groundbreaking findings published in the esteemed journal JAMA provide hope for the possibility of effectively managing and mitigating the effects of this debilitating condition.
The UK’s drugs watchdog is already in the process of assessing donanemab for potential use within the National Health Service (NHS). This development signals the recognition of its potential to make a genuine difference in the lives of Alzheimer’s patients.
It’s important to note that donanemab’s effectiveness is specific to Alzheimer’s disease and does not extend to other forms of dementia, such as vascular dementia.
During the trials, the drug demonstrated a remarkable capacity to slow the progression of Alzheimer’s, resulting in an approximate one-third reduction in the pace of the disease. This deceleration allows individuals to maintain a higher level of independence and preserve their day-to-day activities, including preparing meals and pursuing hobbies.
In conclusion, the promising results from the donanemab trials offer a glimmer of hope in the fight against Alzheimer’s, suggesting that this breakthrough medication could significantly enhance the quality of life for those affected by this challenging condition. As further research and assessments continue, there is a renewed sense of optimism within the medical community and among patients and their families, looking forward to a future with improved Alzheimer’s treatment options.
Sign up to receive our email updates
By submitting this form I agree to the ADSS privacy policy, details of which can be found here
Contact Us
Alzheimer’s and Dementia is a registered charity in England and Wales (1173379). A company limited by guarantee and registered charity in England and Wales (10690071). Registered address: Safeharbour, Coldharbour Road, Northfleet, Kent DA11 8AE
Follow us on social media